ExiTron™ P is a polymeric nanoparticle based CT contrast agent designed for preclinical angiography with renal excretion capability. The formulation consists of iodine containing nanometer sized polymeric capsules, providing prolonged intravascular residence and stable vascular contrast in small animal models. Ordering through an authorized distributor ensures reliable supply, regulatory compliant shipping, and access to technical support for renal and vascular imaging studies.
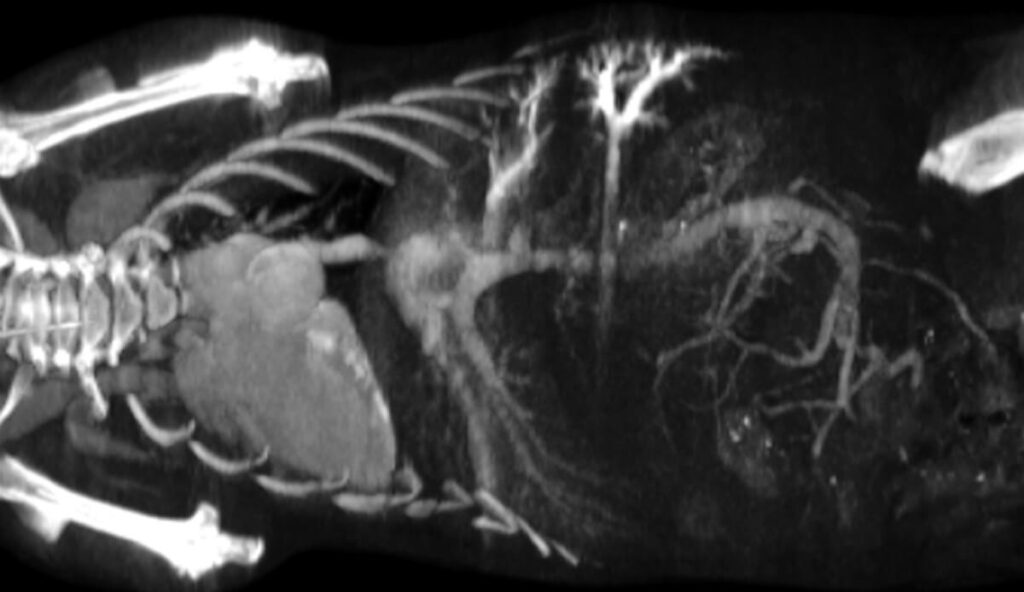
Conventional CT agents often exhibit rapid clearance, limiting imaging of fine vascular structures and renal function. ExiTron™ P addresses this limitation by combining extended blood circulation with dual renal and hepatobiliary elimination pathways. This dual clearance supports visualization of both systemic vasculature and renal structures, enabling comprehensive preclinical studies of vascular physiology, renal function, and pathophysiological changes.
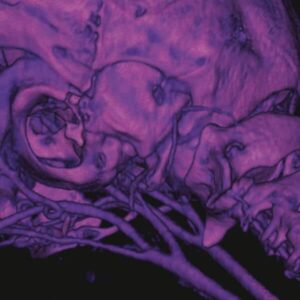
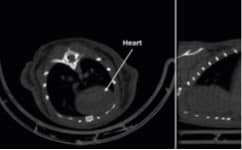
Following administration, ExiTron™ P produces strong and sustained enhancement of the blood pool, facilitating detailed visualization of healthy vasculature and regions of vascular abnormality, such as tumor or inflammation associated microvasculature. The renal excretion property allows imaging of the kidneys and urinary tract, supporting functional and structural assessment over extended time periods.
ExiTron™ P is applied in small animal CT studies requiring high resolution angiography, detection of vascular abnormalities, and renal imaging. Its extended circulation time allows flexible acquisition timing, while dual elimination supports longitudinal evaluation of vascular and renal physiology and pathology.
ExiTron™ P consists of polymeric nanoparticles engineered for blood pool imaging and renal excretion. The mean hydrodynamic diameter is approximately 290 nanometers. Undiluted X ray attenuation reaches approximately 8000 Hounsfield units, providing robust contrast for preclinical vascular and renal CT imaging.
Preclinical studies demonstrate clear whole body vascular enhancement and renal contrast following ExiTron™ P administration. These findings support reproducible visualization of vascular structures and kidney imaging, suitable for quantitative analysis and longitudinal studies.
The application of ExiTron™ P has been documented in preclinical research, including studies on renal imaging and vascular contrast enhancement in murine models.
ExiTron™ P is available in packaging formats suitable for exploratory or repeated studies, including one set of five injections or five sets of five injections. Ordering through an authorized distributor provides access to local support, institutional purchasing assistance, and custom quotations.
Supporting documentation includes a detailed product data sheet outlining formulation properties, recommended use, and imaging guidance for vascular and renal CT studies.
Authorized distributor of Viscover products by Nanopet Pharma GmbH. This page contains original distributor specific content intended to support preclinical CT angiography and renal imaging research.
USD $551 – USD $2,546Price range: USD $551 through USD $2,546