Eye Tracking as a Cost-Effective Tool in Neuroscience Research
Almost all animals with functional vision exhibit a variety of eye movements. These movements allow compensation for shifts in the visual scene and enable tracking

MediLumine is honored to be named a finalist for the 2025 World Molecular Imaging Congress (WMIC) Commercial Innovation of the Year Award. The nomination recognizes the development of a NIST low-power LED light source designed to enable reproducible calibration and sensitivity characterizations of in vivo imaging systems.
This recognition underscores the importance of standardized methodologies in molecular imaging. By providing a reliable reference for photon flux, our innovation addresses long-standing challenges in sensitivity assessment and instrument comparison within the preclinical imaging community.
Bioluminescent imaging (BLI) has become a widely adopted modality for non-invasive monitoring of biological processes in living animals. However, its utility has been constrained by the absence of standardized approaches to measure and compare system performance.
Despite the high cost, cooled CCD cameras are still often considered the gold standard by researchers, underscoring the need for more cost-effective alternatives. Although cooled CCD cameras are still treated as the reference point in preclinical imaging, their expense and outdated design limit broader adoption. Using our NIST-calibrated light source, we validated the PRISM BIO-BOX powered by a Sony CMOS sensor, which has already shown sensitivity down to ~3,000 photons/s/cm²/sr. Until now, the lack of a traceable calibration tool has made it difficult to fairly make such comparisons.
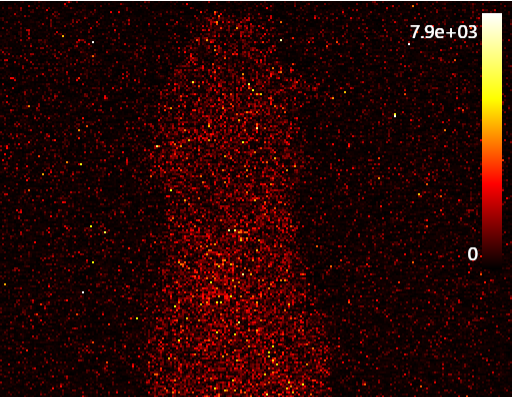

MediLumine’s portable 475 nm LED light source was developed to provide a standardized, NIST-traceable method for optical calibration. Verification was performed using a Thorlabs PM100D power meter equipped with an independently calibrated NIST photodiode sensor, confirming reliable performance across a dynamic range exceeding 10⁷. This wide dynamic range is achieved by stacking neutral density (ND) filters, enabling precise control of emission intensity from strong bioluminescent levels down to near single-photon flux. The optical delivery pathway was designed to reproduce realistic imaging geometries, ensuring that calibration results translate directly to in vivo experimental conditions.
Battery operation allows stability independent of mains fluctuations, and stacking neutral density filters enables researchers to fine-tune photon flux with precision and mimc intensities in the range of autoluminescence. The system therefore establishes a reliable benchmark for both routine quality assurance and inter-platform comparisons.
When applied in comparative testing, the LED light source revealed that CCD-based systems were up to 100-fold less sensitive than the PRISM in vivo imaging platform. For the first time, researchers can quantify sensitivity and background noise using standardized photon flux values expressed in photons per square centimeter per second per steradian. This approach moves the field away from performance claims that lack independent validation and toward reproducible, data-driven decision making.
The impact of this work extends across the imaging community. Facilities can now confirm whether legacy CCD systems remain adequate, benchmark the performance of CMOS versus CCD platforms, and make procurement choices supported by quantitative evidence. Ultimately, this innovation promotes reproducibility, sensitivity, and alignment with the 3Rs of animal research.
Reaching finalist status at WMIC 2025 validates MediLumine’s mission to provide the molecular imaging community with quantitative tools that strengthen research outcomes. This project has been made possible through the contributions of Stephen Marchant at MediLumine Inc., Maxime Abran at Labeo Technologies Inc., and Alexandra De Lille at Perseid LLC.
We look forward to sharing further updates during WMIC 2025 and to continuing our work in advancing transparent and standardized evaluation methods for imaging technologies.
See light source product page here : https://medilumine.com/product/nist-calibrated-low-power-led-light-source
Almost all animals with functional vision exhibit a variety of eye movements. These movements allow compensation for shifts in the visual scene and enable tracking

Medilumine is pleased to announce a new distribution partnership with NanoPET Pharma through its Viscover brand. Medilumine is now an authorized distributor of the full